Carbon dioxide is a very important gas that we use in our daily life. This gas is composed of two non-metallic elements.
As you may know, carbon and oxygen atoms are joined together by a covalent bond in carbon dioxide. But why is co2 creating covalent bonds instead of ionic bonds? In this tutorial, we will discuss this topic. Let us know more!
Why does Co2 form covalent bonds instead of ionic bonds?
There are many reasons behind the formation of covalent bonds of Co2, some of which are important
i. To make ionic bonds, there must be a lot of electro-negative differences between the two atoms. However, there is no such difference between carbon and oxygen because both are non-metallic.
ii. The last cell of carbon is located very close to the nucleus and the size of the nucleus is relatively small. For this reason, in the case of carbon, it is not easy to octate by eliminating or accepting four electrons.
Therefore, carbon four electrons form covalent bonds by mutual sharing with oxygen.
How does Co2 form a covalent bond?
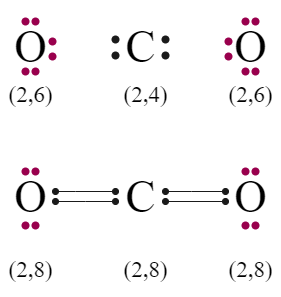
To understand Co2 bond formation, you must first understand the electronic configuration of carbon and oxygen. The electronic configuration of Carbon is 1S2 2S2 2P2 i.e. the last cell of Carbon has 4 electrons. And in the case of an oxygen atom, the electronic configuration is 1S2 2S2 2P4, which means that it would take two more electrons to fill the octet of oxygen.

To fill eight electrons in the last cell of carbon still needs four electrons. For stable conditions, carbon mutually shares four electrons with two oxygen atoms to form two pairs of covalent bonds. As a result, the outer cell of two oxygen atoms is filled with eight electrons.
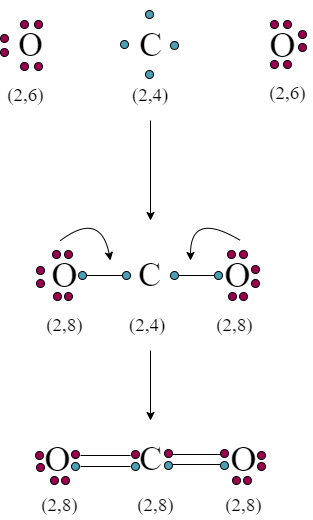
Lewis dot structure of carbon dioxide (Co2)
Valency cell of carbon and oxygen has four and six electrons. However, after the formation of carbon dioxide, there are no electrons on the carbon. But, two lone peer electrons are present on each oxygen.