There are many reasons behind the formation of covalent bonds instead of ionic bonds of carbon dioxide (co2).
C and O are both electro-negative elements
Ionic bonds are always formed between metal and non-metal. Metal is a highly electro-positive element that can easily give electrons to other electro-negative elements.
However, in this case both carbon and oxygen are electro-negative elements or non-metals. Thus, both have higher tendency to receive electrons.
Distance of last cell from the nucleus of carbon is very short
Many of you think that carbon is the last electro-negative element. So, carbon will form an ionic bond with two oxygen atoms with four electrons.
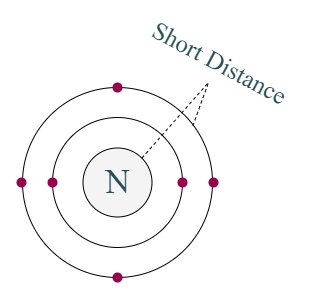
But no, carbon cannot easily give four electrons because the distance between last cell and nucleus is less. So, the force of attraction will be too much.
Nucleus cannot manage the extra four electrons
Even if the octet is filled with four extra electrons, the six protons of carbon cannot manage the extra four electrons.
For these three reasons, covalent bonds are formed between carbon and oxygen instead of ionic bonds.